RESORBABLE, 100% SYNTHETIC SURGICAL MESH
TIGR® Matrix is the world’s first long-term resorbable, 100% synthetic, surgical mesh. Its unique technology consisting of dual-stage degradation and full resorption, paired with ease of use, is a significant step forward in surgical mesh technology. Since it uses polymers common in medical devices since the 1970’s, and is 100% synthetic, its components are more than well documented and clinically proven.
use in many applications
Today, TIGR®Matrix finds use in many applications, e.g. breast reconstruction after cancer, breast augmentation revision and abdominal wall closure. Please visit the evidence section to view currently available clinical evidence. Before using TIGR® Matrix read the instructions for use which accompany the product for indications, contraindications, warnings and precautions.
TIGR®Matrix Surgical Mesh received 510(k) clearance by the FDA in 2010 and carries the CE-mark since 2011.
available in 3 sizes
- NSTM1015E, 10 x 15 cm
- NSTM1520E, 15 x 20 cm
- NSTM2030E, 20 x 30 cm
TECHNOLOGY
TIGR® Matrix was developed to take advantage of the process whereby mechanical load induces remodeling of soft tissue, termed dynamic remodeling. After initial wound closure, the increasing compliance of the mesh results in a gradual transition of load from the mesh to the tissue. Long-term strength retention combined with the dynamic remodeling-based design opens up new opportunities in soft tissue repair.
MECHANICS
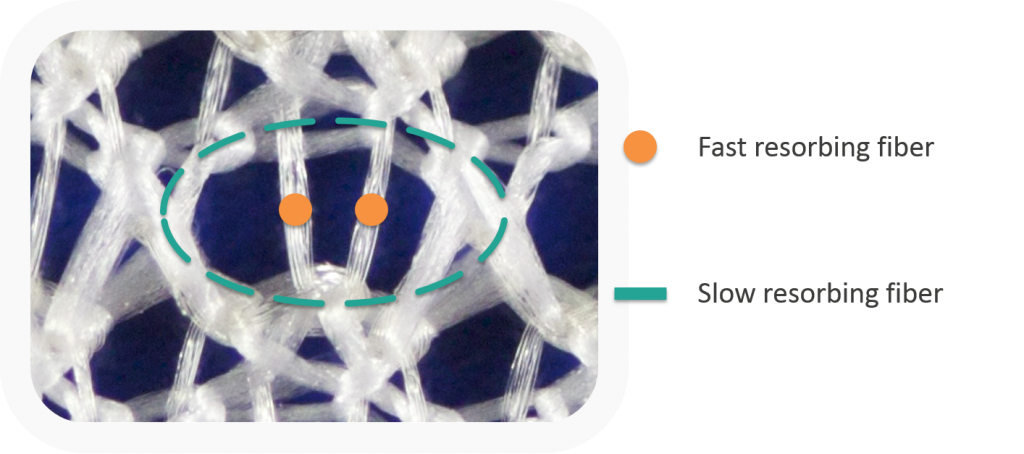
Multistage mechanics are achieved by arranging two fibers with different degradation characteristics in an interlocking knitting pattern.
- Strength and stability of the mesh is high in the initial wound-healing phases (closure and granulation).
- Macro porosity throughout the mesh allows for good integration during granulation.
- As the granulation phase transitions into the remodeling phase, the elasticity of the mesh gradually increases.
- Movement dynamics during remodeling stimulates the regeneration of tissue.
- The result of this dynamic remodeling is a more structured, and hence stronger, connective tissue.
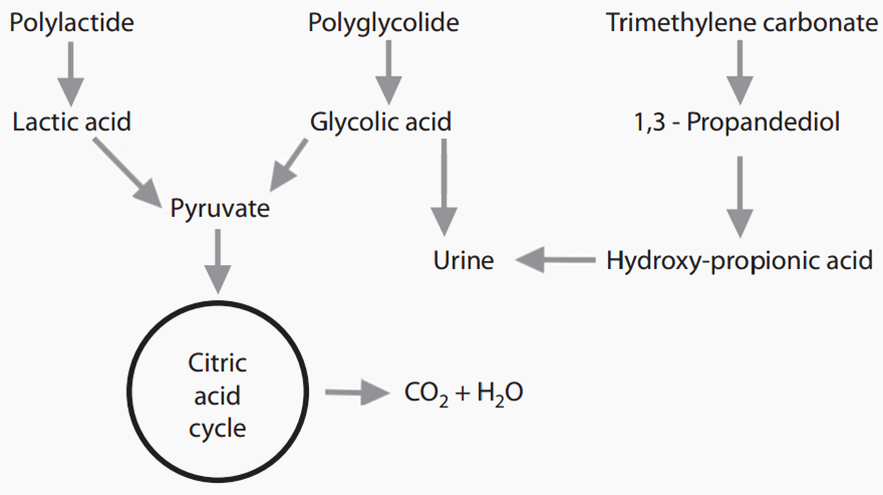
MATERIALS
-
Locks the knitting pattern during initial wound-closure
-
Loses mechanical strength in a couple of weeks
-
Completely resorbs in 4 months
-
Copolymer of glycolide, lactide and trimethylene carbonate
-
Acts as tissue reinforcement during remodeling
-
Strong for at least 6 months
-
Completely resorbs in 3 years
-
Copolymer of lactide and trimethylene carbonate
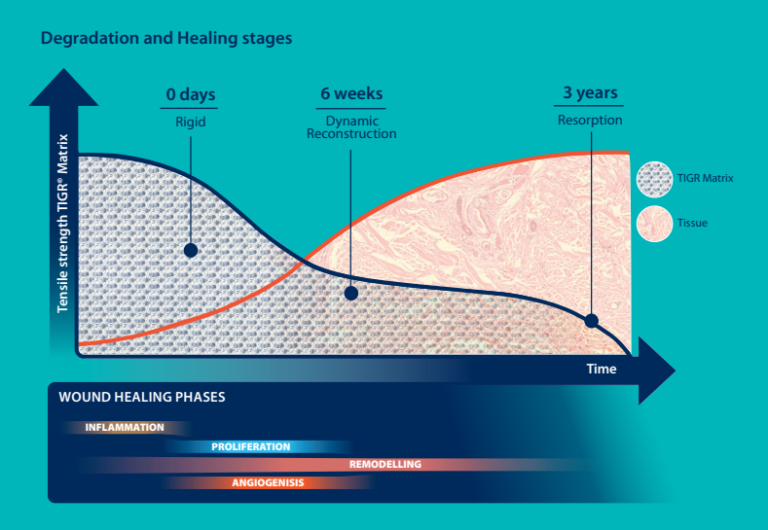
DEGRADATION AND RESORPTION
- Initially, the mesh is rigid owing to the locked knitting pattern and polymer design.
- After a couple of weeks, there is no strength left in the fast resorbing fibers. However, the long-term resorbable fibers remain to reinforce the tissue with a more elastic characteristic
- Three years after implantation, there is no trace left of the mesh.
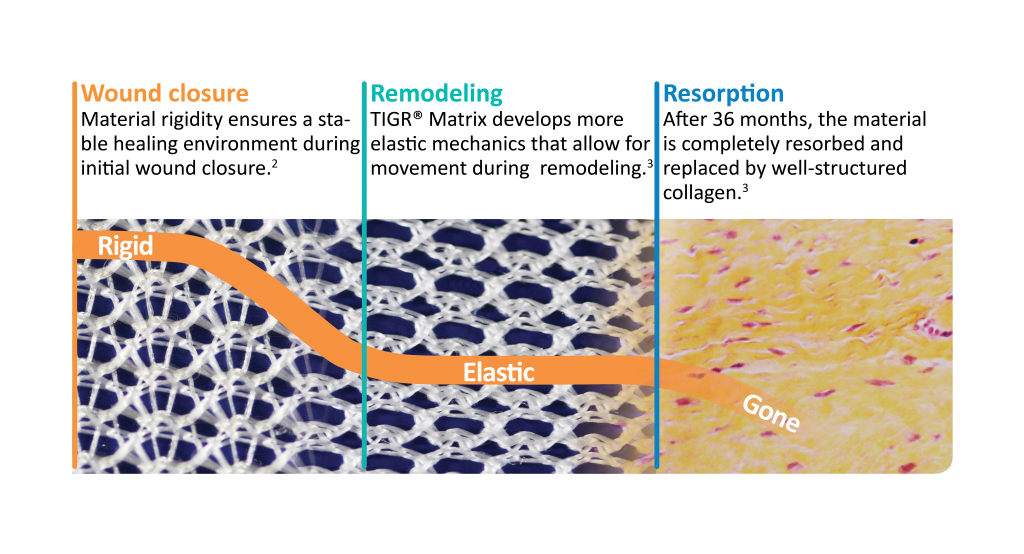
PROCESS
STERILIZATION
TIGR® Matrix is sterilized with ethylene oxide (EtO) and is for single use only.
PACKAGING
- The mesh is sterile.
- The outside of the inner pouch is non-sterile.
- Made of Tyvek® and polyethylene.
- The outer pouch prevents moisture from coming into contact with the mesh.
- The atmosphere in the outer pouch is extremely dry.
- Made of aluminum.
- Schultz S. et al. 2005, World Wide Wounds; Kingsnorth N. et al. 2013, Management of Abdominal Hernias; Junge U. et al. 2001, Hernia
- Data on file, in vitro resorption.
- Three-year results from a preclinical implantation study of a long-term resorbable surgical mesh with time-dependent mechanical characteristics H. Hjort, T. Mathisen, A. Alves, G. Clermont, J. P. Boutrand, Hernia, 16(2):191–197, 2012